Improving Lives By Protecting Hearing
Spiral is advancing a therapeutic pipeline with focus on balance disorders and hearing loss. Our lead program, SPT-2101, is a sustained-release steroid formulation for the treatment of Ménière’s disease.
About
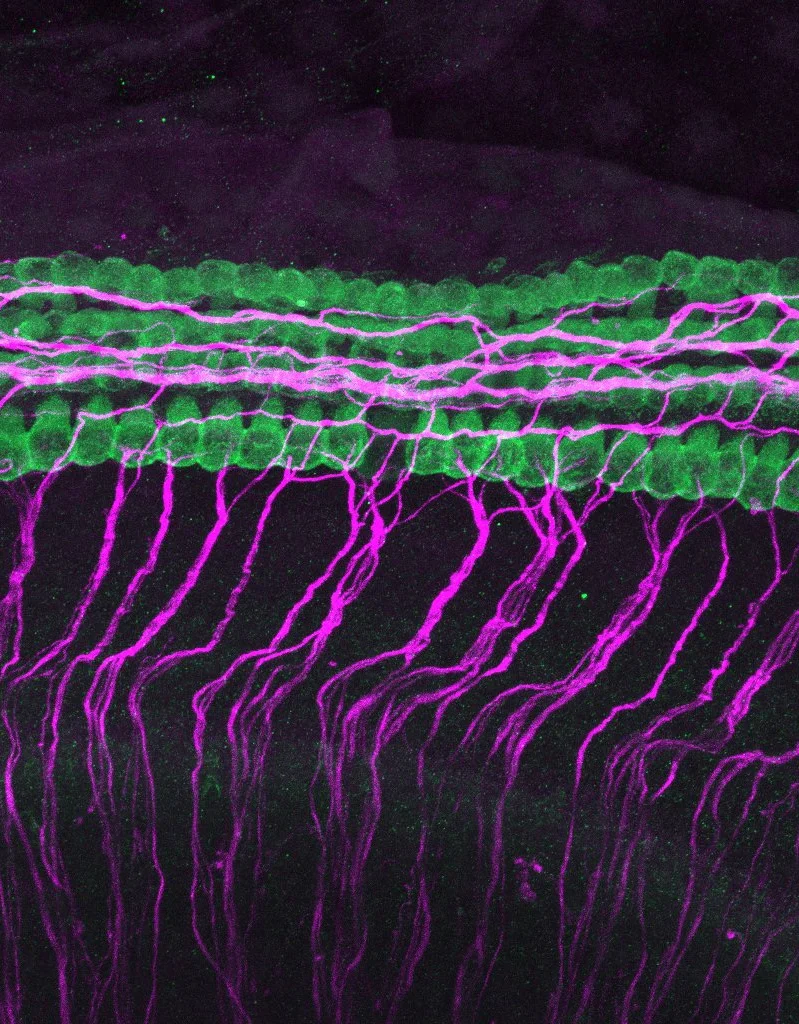
40 million people in the US qualify for hearing loss treatment, but there are no drugs approved for hearing loss. Drug delivery to the inner ear remains the largest obstacle.
Precise, non-invasive placement techniques, combined with long-acting delivery systems have the potential to overcome the barriers of drug delivery to the inner ear.
Inflammation and neurodegeneration are the origin of 90% of inner ear disorders. Biomarkers of active disease and improved patient selection are necessary to overcome the challenges of clinical development.
Inspired by ophthalmology, Spiral’s platform addresses hearing loss by delivering the right drugs, to the right place, for the right duration. Our novel drug delivery platform allows for minimally invasive, precise and durable exposure of drugs to the cochlea.
Spiral is advancing a therapeutic pipeline with focus on balance disorders and hearing loss. Our lead program, SPT-2101, is a sustained-release steroid formulation for the treatment of Ménière’s disease.
Leadership
Board of Directors

Hugo Peris
FOUNDER & CEO

Angela Macfarlane
INDEPENDENT

Guy Jean Savoir
SAVOIR CAPITAL

Tony Adamis
INDEPENDENT

Eugene de Juan
EXECUTIVE CHAIRMAN

Fred Guerard
INDEPENDENT

Diamantis Xylas
CATALIO CAPITAL
Technology
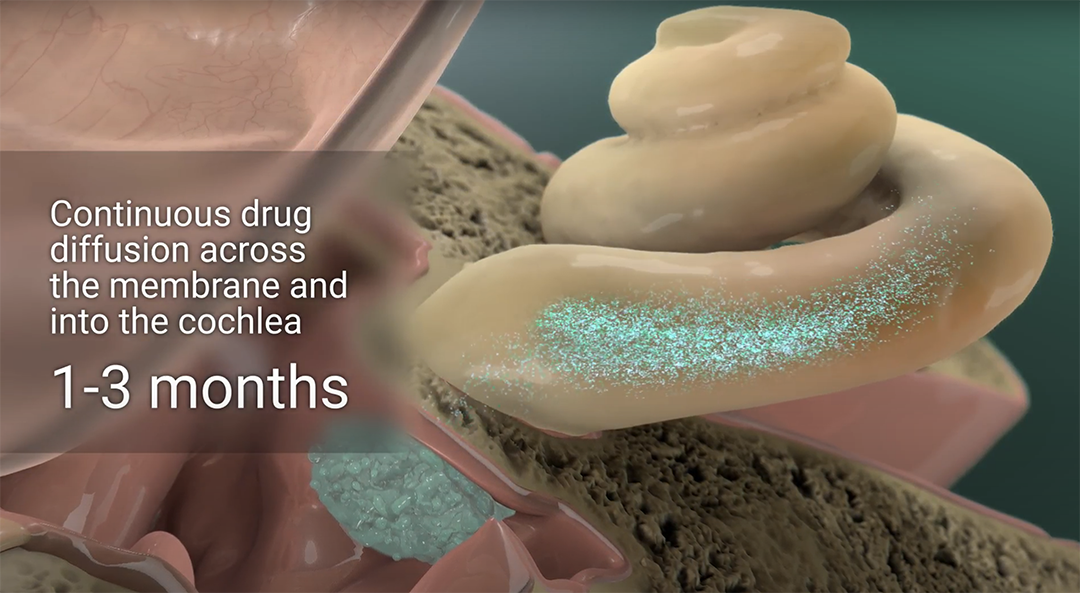
Spiral’s MICSTM (minimally-invasive cochlear system) delivery platform is uniquely suited to deliver a wide range of drugs to the ear, with high precision and long duration, in a clinic-friendly approach. Our formulations achieve weeks to months of residence in the middle ear, and have been adapted to deliver drugs with anti-inflammatory, otoprotective and/or neuroprotective activity for the treatment of balance disorders and hearing loss.
Ménière's disease
Ménière’s disease is a chronic condition characterized by acute vertigo attacks, tinnitus, fluctuating hearing loss and a feeling of aural fullness. Of these symptoms, the vertigo attacks are typically most troubling for patients since they disrupt daily activities and are difficult to anticipate and manage. In general, patients are diagnosed with unilateral Ménière’s disease in middle age and symptoms often continue for decades. Over time, the fluctuating hearing loss becomes permanent in many patients, and a subset of patients will develop symptoms in their second ear. According to the National Institute of Deafness and Other Communication Disorders, there are more than 600,000 patients diagnosed with Ménière’s disease in the United States. There is no known cure for Ménière’s disease and there are currently no FDA-approved drug treatments.
Our lead program, SPT-2101 (6% dexamethasone formulated for sustained release), recently completed a Phase 1b/2a clinical trial in Ménière’s disease.
Using Spiral’s proprietary Minimally Invasive Cochlear System (MICS™) platform in the clinic, SPT-2101 was delivered precisely to the round window membrane for extended release in all study participants. No serious adverse events (SAEs) or unexpected adverse events (AEs) were reported, and no negative impact on hearing was observed. All patients experienced full resolution of the myringotomy.
The treatment with SPT-2101 showed superior vertigo management compared to the control group, with a statistically significant difference (p< 0.05). Additionally, non-responders in the placebo group who crossed over to receive SPT-2101 demonstrated a dramatic reduction in Definitive Vertigo Days at Month 3 (78.7%). The data suggest higher dexamethasone exposures correlated with improved vertigo management across the treatment period, regardless of baseline disease severity.
News
Spiral Therapeutics Announces Successful Completion of the SPT-2101 PHASE 1b/2a Clinical Trial for Meniere’s Disease, Data Presented During the 2024 AAO Annual Meeting
SOUTH SAN FRANCISCO, Calif., Sept. 30, 2024 -- Spiral Therapeutics, Inc. (Spiral), a clinical-stage company
Spiral Therapeutics To Present At The Barany Society Meeting 2024
SOUTH SAN FRANCISCO, Calif., Aug. 21, 2024 -- Spiral Therapeutics, Inc. (Spiral), a pharmaceutical company
Spiral Therapeutics to Participate at the 2024 Wedbush PacGrow Healthcare Conference
SOUTH SAN FRANCISCO, Calif., Aug. 5, 2024 -- Spiral Therapeutics, Inc. (Spiral), a pharmaceutical company dedicated









