Improving Lives By Protecting Hearing
Spiral is advancing a therapeutic pipeline with focus on balance disorders and hearing loss. Our lead program, SPT-2101, is a sustained-release steroid formulation for the treatment of Ménière’s disease.
About
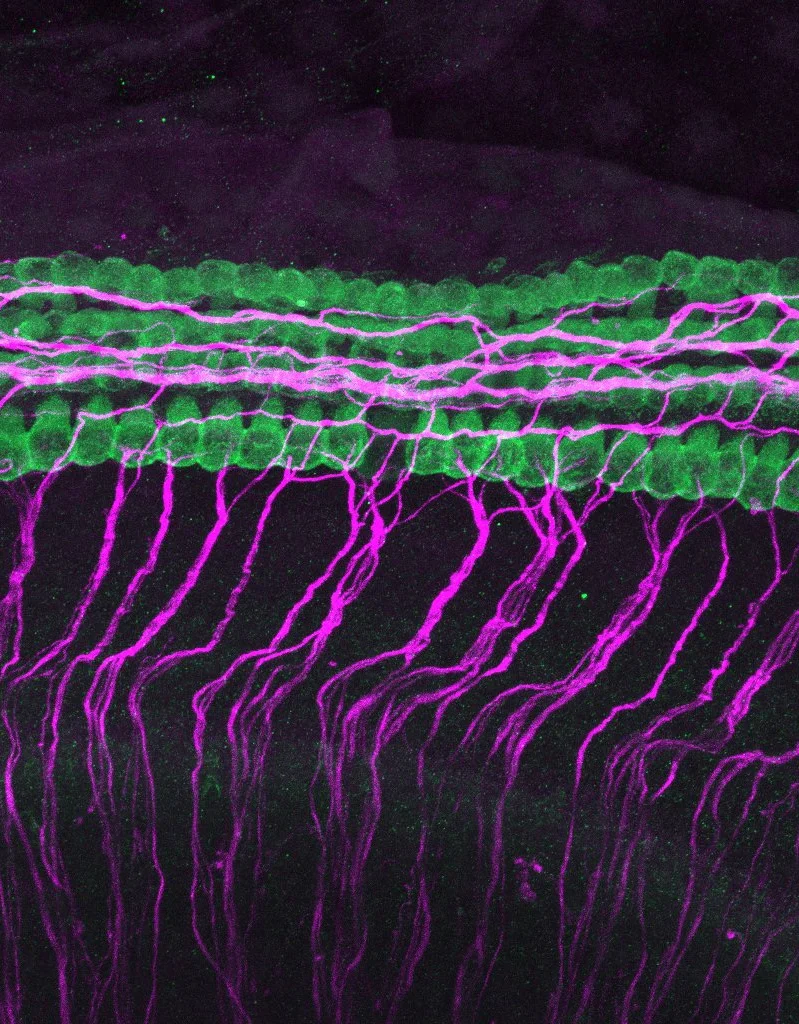
40 million people in the US qualify for hearing loss treatment, but there are no drugs approved for hearing loss. Drug delivery to the inner ear remains the largest obstacle.
Precise, non-invasive placement techniques, combined with long-acting delivery systems have the potential to overcome the barriers of drug delivery to the inner ear.
Inflammation and neurodegeneration are the origin of 90% of inner ear disorders. Biomarkers of active disease and improved patient selection are necessary to overcome the challenges of clinical development.
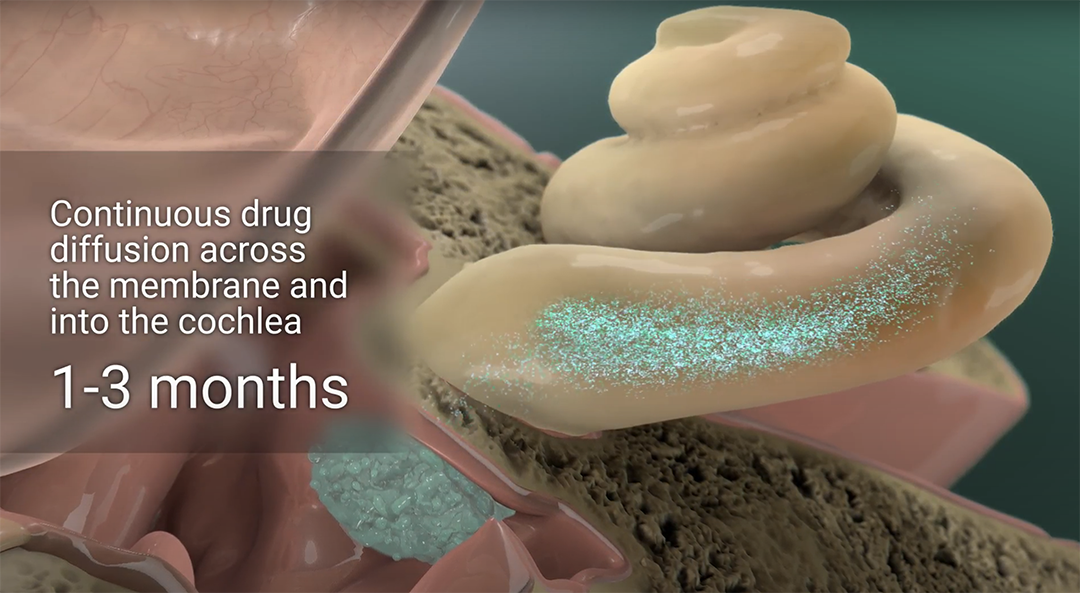
Inspired by ophthalmology, Spiral’s platform addresses hearing loss by delivering the right drugs, to the right place, for the right duration. Our novel drug delivery platform allows for minimally invasive, precise and durable exposure of drugs to the cochlea.
Spiral is advancing a therapeutic pipeline with focus on balance disorders and hearing loss. Our lead program, SPT-2101, is a sustained-release steroid formulation for the treatment of Ménière’s disease.
Team
Executive Team
Board of Directors

Hugo Peris
FOUNDER & CEO

Angela Macfarlane
INDEPENDENT

Guy Jean Savoir
SAVOIR CAPITAL

Tony Adamis
INDEPENDENT

Eugene de Juan
EXECUTIVE CHAIRMAN

Fred Guerard
INDEPENDENT

Diamantis Xylas
CATALIO CAPITAL
Technology
Ménière's disease
Ménière’s disease is a chronic condition characterized by acute vertigo attacks, tinnitus, fluctuating hearing loss and a feeling of aural fullness. Of these symptoms, the vertigo attacks are typically most troubling for patients since they disrupt daily activities and are difficult to anticipate and manage. In general, patients are diagnosed with unilateral Ménière’s disease in middle age and symptoms often continue for decades. Over time, the fluctuating hearing loss becomes permanent in many patients, and a subset of patients will develop symptoms in their second ear. According to the National Institute of Deafness and Other Communication Disorders, there are more than 600,000 patients diagnosed with Ménière’s disease in the United States. There is no known cure for Ménière’s disease and there are currently no FDA-approved drug treatments.
Our lead program, SPT-2101, is currently the subject of an investigational clinical trial in Australia for the treatment of Ménière’s disease. Early data from this trial is showing significant improvement in the frequency of patient reported vertigo episodes and other efficacy outcome measures.
News
Spiral Therapeutics Receives Hearing Technology Innovator Award
Spiral has been recognized for its innovative Drug Delivery Platform in the fourth
Spiral Therapeutics Announces Collaboration with the Vestibular Disorders Association (VeDA), Advancing Patient-Centric Care in Neurotology
Partnership with VeDA enhances Spiral's commitment to patient outreach and education, while gaining
Spiral Therapeutics Welcomes New Investment, Cementing Its Leadership in Neurotology
Investment led by Esperante Ventures and Ferring Ventures: Additional funding to support expanded SPT-2101









